
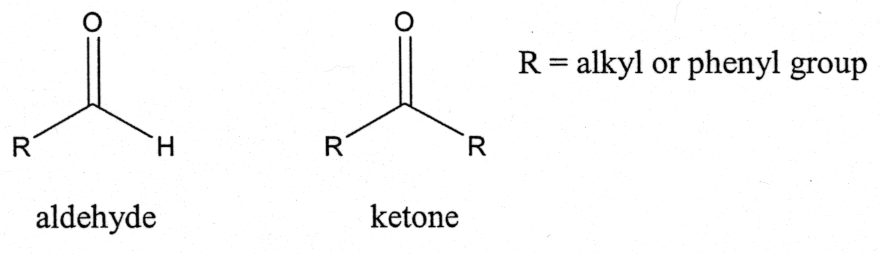
Figure 2.3h Primary, secondary, & tertiary amineįor the functional groups in the 2 nd part of Table 2.2, they all have a common structural unit of a carbonyl group C=O the different structure of “W” in the general formula determines the nature of the functional group. Amines can also be referred to with common names. Amine can be primary (1°), secondary (2°) or tertiary (3°) depending on how many R groups are connected with nitrogen. When the hydrogen atom(s) in NH 3 is replaced with R groups, it produces amine. Nitrile has a C≡N triple bond, and therefore can only be at the end of a structure, while nitro (NO 2) can be in any position on the carbon chain or ring.Īmine is the organic derivative of ammonia, NH 3. Figure 2.3g Cyclic ether examplesīoth nitrile and nitro groups contain nitrogen atoms, and they can be easily confused. It may not be that intuitive to recognize the following structure as ether, and labelling the carbon atom will be helpful for identification. Figure 2.3f diethyl etherĮther can be in a cyclic structure as well. When the two alkyl groups are the same, they can be combined as “dialkyl”. Compounds with ether as the only functional group are usually referred to with the common name “alkyl alkyl ether”. In ether, the O atom connects with two carbon-containing R groups through two C-O σ bonds. Figure 2.3e 1° alcoholĪnother functional group that contains the oxygen atom in single bonds is ether. Depending on the position of the OH group, alcohols can also be categorized as primary (1°), secondary (2°) or tertiary (3°). In organic chemistry, the term alcohol refers to a compound containing the OH (hydroxy) group. Alcohol is a functional group that you are probably familiar with.


 0 kommentar(er)
0 kommentar(er)
